Editor’s Note:
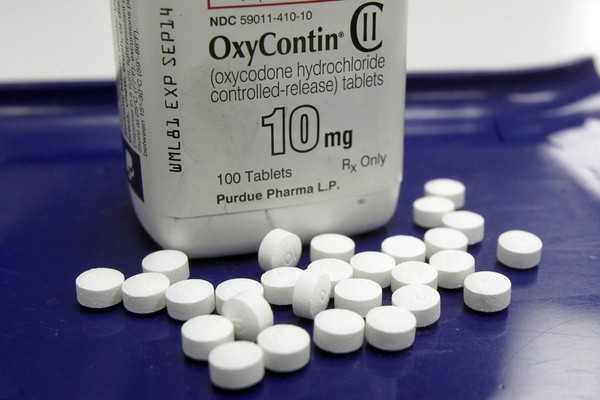
The maker of OxyContin said it will stop marketing opioid drugs to doctors, bowing to a key demand of lawsuits – including one filed by the state of New Hampshire – that blame the company for helping trigger the current drug abuse epidemic. Below is an open letter from Purdue Pharma, that accompanied the surprise reversal on Feb. 10. Purdue’s statement said it eliminated more than half its sales staff this week and will no longer send sales representatives to doctors’ offices to discuss opioid drugs. Its remaining sales staff of about 200 will focus on other medications.
 Two doctors founded a company in 1892 now known as Purdue Pharma. Continuing the strong heritage of a research-driven, science-based company, another doctor is currently at the helm as CEO. We’re the pharmaceutical company that manufactures OxyContin®.
Two doctors founded a company in 1892 now known as Purdue Pharma. Continuing the strong heritage of a research-driven, science-based company, another doctor is currently at the helm as CEO. We’re the pharmaceutical company that manufactures OxyContin®.
Patients’ needs and safety have guided our steps. It’s what led us to research and develop medications to help patients. Today, it’s what has spurred us to redouble our efforts in the fight against the prescription and illicit opioid abuse crisis. It’s why we’re taking action.
We support recommendations in The President’s Commission on Combating Drug Addiction and the Opioid Crisis and the FDA’s Opioid Action Plan. There are too many prescription opioid pills in people’s medicine cabinets. We support initiatives to limit the length of first opioid prescriptions. Reducing the number of excess tablets won’t end the epidemic, but we believe it will help rein in the problem. We believe doctors should check their state Prescription Drug Monitoring Program (PDMP) databases before writing an opioid prescription, to guard against doctorshopping by those trying to game the system. Information sharing between state databases must improve.
Our industry and our company have and will continue to take meaningful action to reduce opioid abuse. We focused our talented research scientists and applied our innovative thinking to making opioids with abuse-deterrent properties, making them harder to crush and, therefore, harder to be abused by snorting or injection. With this investment, we pioneered the pharmaceutical industry’s movement toward developing opioids with abuse-deterrent properties when we were the first to receive FDA approval.¹ (see note below).
Developing new formulations is risky and there are never any guarantees, but we did it anyway. Our company also took the initiative to distribute the CDC Guideline for Prescribing Opioids to thousands of prescribers and pharmacists shortly after it was released. As we continue to fight the prescription opioid and illicit substance abuse crisis, we are applying our resources and our best scientific minds to discover and develop new, non-opioid pain medicines for patients. No one solution will end the crisis, but multiple, overlapping efforts will. We want everyone engaged to know you have a partner in Purdue Pharma. This is our fight, too.
Open Letter from Perdue Pharma







